Topics
A. Liquid water and aqueous solutions
|
Liquid water at 298 K
|
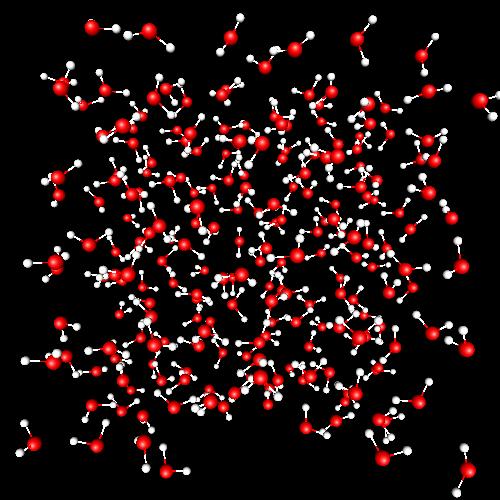 |
|
A tetrahedral network of water molecules is the main structural trend, due to the existence of four hydrogen-bonds per molecule (on average). The lifetime of such hydrogen-bonds is around 1 ps. Hydrogen-bonding is the main responsible of the amazing properties of liquid water. This part of my research is carried out in collaboration with Joan Àngel Padró (University of Barcelona) and Elvira Guàrdia (UPC). |
B. Supercritical water
|
Structure of supercritical water at 723 K.
|
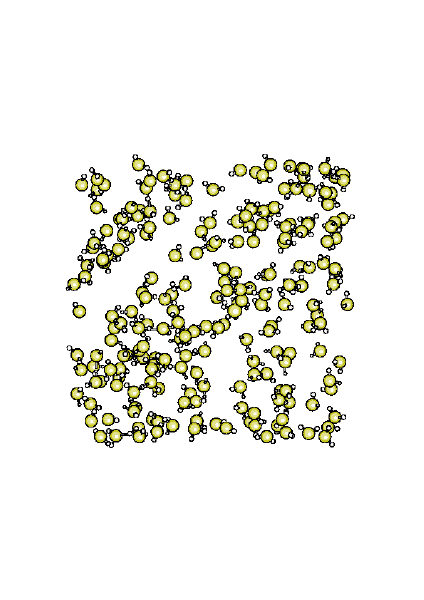 |
|
Water beyond its critical point (T > 647.13 K, d > 0.322 g/cm³, P > 220.55 bar) shows surprising new properties, like the capacity of mixing with oil. In supercritical water there is no longer distinction between the liquid and vapor phase. We have observed the breakdown of the tetrahedral structure and its substitution by cavities and water clusters. The lifetime of hydrogen-bonds is now about 0.3-0.5 ps. This part of my research is carried out in collaboration with Elvira Guàrdia (UPC). |
C. Rare events in chemical physics and biophysics
|
Lipid chain performing a flip-flop transition in a biological membrane
|
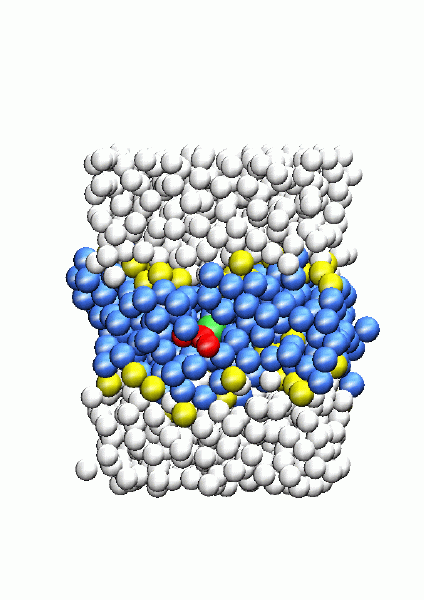 |
|
The study of rare events is a computational challenge due to the tiny amount of trajectories in phase space which lead to such phenomena, compared with the total amount of trajectories described by a microscopical system. A new technique named transition path sampling, which is able to analyze reactive trajectories in configurational space without preconceived information about potential energy surfaces or transition states of the system, has been recently developped by the research group of David Chandler in the University of California at Berkeley. We have employed that technique to study the transition state structure of NaCl dissociation in water and, more recently, flip-flop transitions of lipids in biomembranes. That reserach has been done in collaboration with David Chandler (University of California, Berkeley) concerning NaCl dissociation and also Félix S. Csajka (Max-Plank Institute for Colloids and Interfaces, Potsdam) about NaCl dissociation and lipid transitions. |
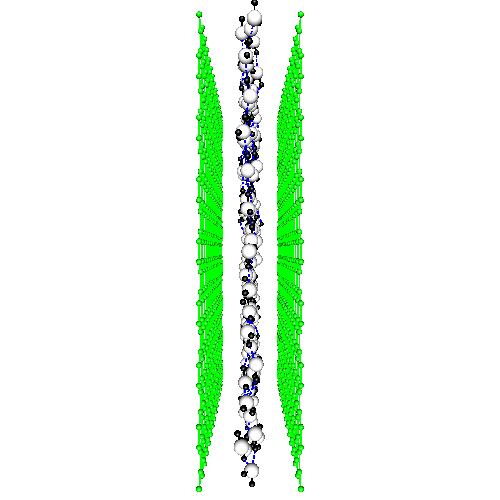
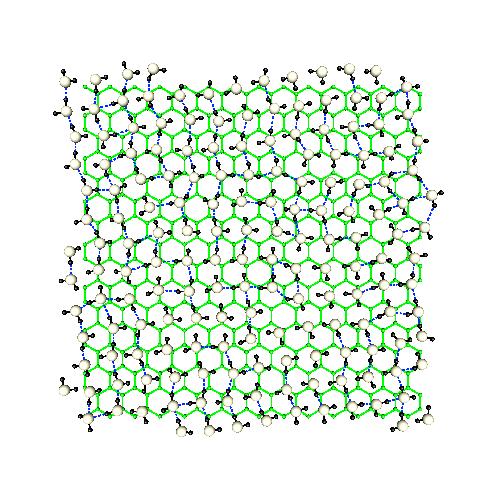
D. Liquid and supercritical water under confinement
|
Liquid water at 298 K adsorbed in a carbon nanotube of radius 40 nm
|
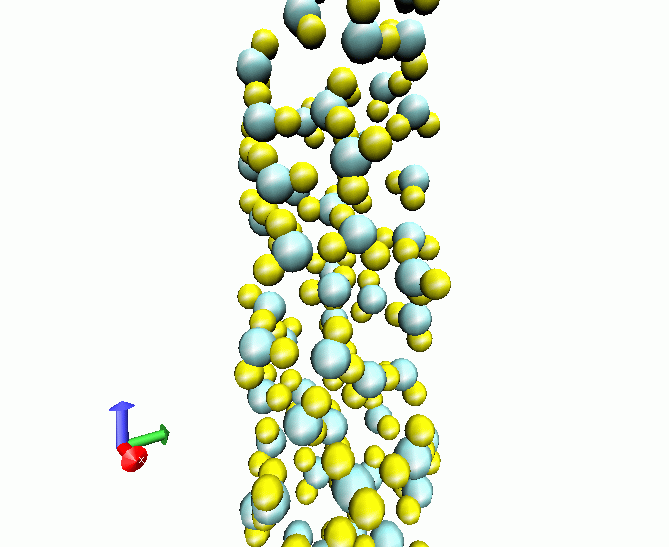 |
|
When liquids are constrained inside solid devices, its behavior can suffer dramatic changes. In the case of water, confinement produces marked loss of structural order and modifications in its microscopic dynamics. We have simulated several liquid and supercritical water samples when constrained by rigid and soft carbon nanotube walls. One of the most relevant cases is that of quasi-one dimensional liquid water, when liquid water is simply composed by |
|
The water-solid interface
|
|
|
As another example of confinement, we have simulated liquid water imbedded in between two flat graphite layers. This is a "classical" system in simulation studies which we will use to learn further about modifications in the structure and dynamics of water under extreme confinement. In the limit, we are able to speak about 2D water.The influence of the tubes is especially relevant concerning hydrogen stretching vibrations and diffussive behavior. This research work is been produced in collaboration with M.C.Gordillo (Pablo de Olavide University at Seville), Gabor Nagy (Atomic Energy Research Institute of the Hungarian Academy of Sciences) and Elvira Guàrdia (UPC) |
|
 |
 |
Share: